{{i.title_en}}
Novel Coronavirus Real Time Multiplex RT-PCR Kit
MOQ: 10000 Set
Note: If you have any questions,please contact us
- 1.Reliable diagnosis: TGA approved ;1st IVD approved by CFDA for detection of 2019-nCoV RNA
- 2. PCR with high sensitivity and specificity, validated with over 200 natural clinical specimens
- 3.Wide range of specimen types: Sputum ;Nasopharyngeal swab; bronchoalveolar lavage; Throat swab
- 4.Multiplex real-time PCR; Detection and identification of target genes(ORFlab, E, N) specific for COVID-19 in a single tube
- Real Time RT-PCR Kit (2019-nCoV)

Shanghai ZJ Bio-Tech Co., Ltd
Since:
2005
Product Description
| Category | Product Name | Cat No | Specifications | Specifications |
| Detection kit | Novel Coronavirus (2019-nCoV)Real Time Multiplex RT-PCR Kit | RR 0479-02 | 25 tests/kit | 1/1TGA approved,1st CFDA approved kit; 2/1 Detecting 3 target genes in a single tube |
| Extraction kits | RNA Isolation Kit | ME 0044/0045 | 60/240 tests/box | Pre-loaded compatible with EX3600,CE marked |
| RNA Isolation Kit | ME 0067/0068 | 80/320 tests/box | Pre loaded compatible with EX1600,CE marked | |
| RNA Isolation Kit | ME 0010/0025 | 50/25 tests/box | Paramagnetic beads column | |
| instruments | Automated Nucleic Acid;Extraction System | EX3600 | 36 samples/run | Automatic nucleic acid extraction,effectively; preventing cross contamination,20 min/run |
| Automated Nucleic Acid;Extraction System | EX 1600 | 16 samples/run | Automatic nucleic acid extraction,effectively; preventing cross contamination,20 min/run | |
| qPCR Cycler | MIC | 48 wells; 6 channels | Lightweight and portable fast and accurate;compatible with car power 60 min/run | |
| AutraMic | AutraMic48 | 48 tests/run | 1/Sample to result; 2/ Confined space with HEPA | |
| Swab | OHC0381 | 100 pieces/bag | For throat swab collection | |
| Sample Collection Device | Sputum Collector/Sample Container | OHC0361 | 100 tubes/bag | For sputum collection can also be used as sample container |
| Virus Transport Medium(VTM)/Lysis Buffer | PR-0020 | 15 tubes/bag | 1/ For virus inactivation; 2/ Protecting RNA from degradation | |
| Virus Transport Medium(VTM)/Lysis Buffer | PR-0021 | 15 tubes/bag | 1/ For liquefaction of sputum; 2/ For virus inactivation;3/ Protecting RNA from degradation |
Novel Coronavirus (2019-nCoV) Real Time Multiplex RT-PCR Kit (Detection for 3 Genes)
Instructions for Use
1. Intended Use
Novel Coronavirus (2019-nCoV) Real Time Multiplex RT-PCR Kit (Detection for 3 Genes) is used for the in vitro qualitative detection of 2019 novel coronavirus (2019-nCoV) RNA in
nasopharyngeal swab, oropharyngeal swab and sputum specimens by real time PCR systems. It is considered as an aid in the diagnosis of the 2019-nCoV infection.
2. Principle of Real-Time RT-PCR
The principle of the real-time detection is based on the fluorogenic 5’nuclease assay. During the PCR reaction, the DNA polymerase cleaves the probe at the 5’ end and separates the reporter dye
from the quencher dye only when the probe hybridizes to the target DNA. This cleavage results in the fluorescent signal generated by the cleaved reporter dye, which is monitored real-time by the
PCR detection system. The PCR cycle at which an increase in the fluorescence signal is detected initially (Ct) is proportional to the amount of the specific PCR product. Monitoring the fluorescence intensities in real time allows the detection of the accumulating product without having to re-open the reaction tube after the amplification.
Real time reverse-transcription polymerase chain reaction (real-time RT-PCR) is used when the starting material is RNA. In this method, RNA is first transcribed into the complementary DNA
(cDNA) by reverse transcriptase from total RNA. The cDNA is then used as a template for the real time PCR.
3. Product Description
Coronaviruses are a large family of viruses, some causing illness in human and others circulating among animals such as camels, cats and bats. 2019-nCoV is a novel coronavirus. The kit contains a specific ready-to-use system for the detection of Novel Coronavirus (2019-nCoV) RNA by the real-time RT-PCR. The reaction is done in a one-step real time RT-PCR assay in a single tube. It includes a reverse transcription (RT) for the transcription of virus RNA into cDNA and real time PCR for the amplification and detection of the cDNA. Fluorescence is emitted and measured by the real time systems´ optical unit during PCR. The detection of amplified virus DNA fragment is
performed in fluorimeter channel FAM, HEX/VIC and Cal Fluor Red610/TEXAS RED.
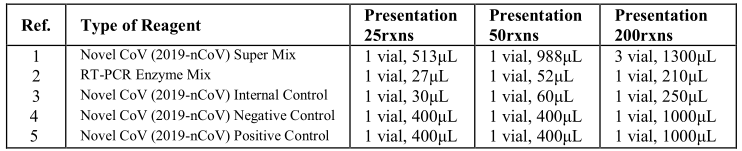
4. Kit Contents
Limit of detection (LoD): 200 copies/mL;
Note: LoD depends on the sample volume, elution volume, nucleic acid extraction method and other
factors. If you use the RNA extraction kits recommended, the LoD is the same as it declares.
However, if you use other extraction method, additional verification should be performed to ensure the analytical performance of the assay.
5. Storage
• All reagents should be stored at -20±5°C. The validity period is 6 months.
• The assay should be used within 1 month after opening.
• Repeated thawing and freezing (> 3x) should be avoided as this may reduce the sensitivity of the assay.
• Cool all reagents during the working steps.
• Super Mix should be stored away from light.
6. Additionally Required Materials and Devices
• Biological cabinet
• Vortex mixer
• Cryo-container
• Sterile filter tips for micro pipets
• Disposable gloves, powderless
• Refrigerator and freezer
• Real time PCR system
• Real time PCR reaction tubes/plates
• Pipets (0.5μL – 1000μL)
• Sterile microtubes
• Biohazard waste container
• Tube racks
• Desktop microcentrifuge for “eppendorf” type tubes (RCF max. 16,000 x g)
7. Warnings and Precautions Carefully read this instructions for use before starting the procedure.
• This assay needs to be carried out by skilled personnel.
• Clinical samples should be regarded as potentially infectious materials and be prepared in a laminar flow hood.
• This assay needs to be run according to Good Laboratory Practice.
• Do not use the kit after its expiration date.
• Avoid repeated thawing and freezing of reagents as this may reduce the sensitivity of the test.
• Once the reagents have been thawed, vortex and centrifuge briefly the tubes before use.
• Prepare quickly the reaction mix on ice or in the cooling block.
• Set up separate working areas for: 1) Reaction setup, 2) Isolation of the RNA and 3)
Amplification/detection of amplification products.
• Pipets, vials and other working materials should not circulate among working units.
• Use always sterile pipette tips with filters.
• Wear separate coats and gloves in each area.
• Discard sample and assay waste according to your local safety regulations.
• Do not pipette by mouth. Do not eat, drink or smoke in laboratory.
• Avoid aerosols
8. Sample Collection, Storage and Transport
• Collect samples in sterile tubes;
• Specimens can be extracted immediately or stored at 2°C~8°C within 24 hours or frozen at -70°C for long-term.
• Transportation of clinical specimens must comply with local regulations for the transport of
etiologic agents
9. Procedure
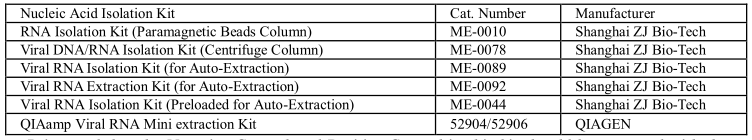
9.1 RNA-Extraction
Different brand RNA extraction kits are available. You may use your own extraction systems or the
commercial kits based on the yield. For the RNA extraction, please follow the manufacturer’s
instructions. The recommended extraction kits are as follows:
It is noted that the Negative Control and Positive Control in this kit should be extracted with the same protocol as for specimens.
9.2 Internal Control
The internal control (IC) in this kit should be added into the extraction mixture with 1μL/test to monitor the whole process.
9.3 RT-PCR Protocol
The Master Mix volume for each reaction should be pipetted as follows:
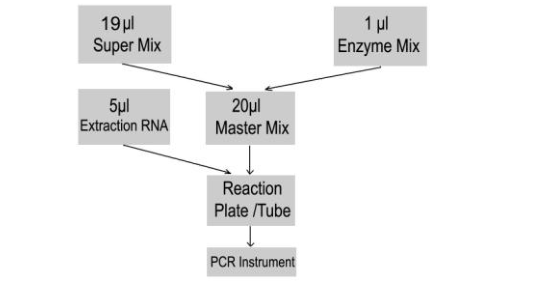
1) The volumes of Super Mix and Enzyme Mix per reaction multiply with the number of
samples, which includes the number of controls and samples prepared. For reasons of
imprecise pipetting, always add an extra virtual sample. Mix completely and then spin down
briefly with a centrifuge.
2) Pipet 20μL Master Mix with micropipets of sterile filter tips to each of the Real Time PCR
reaction plate/tubes. Separately add 5μL template (nucleic acid extracted from negative
control, positive control and specimens) to different reaction plates/tubes. Immediately close
the plates/tubes to avoid contamination.
3) Spin down briefly in order to collect the Master Mix and template in the bottom of the reaction tubes.
4) Perform the following protocol in the instrument of ABI Prism ® 7500; Bio-Rad CFX96; SLAN:
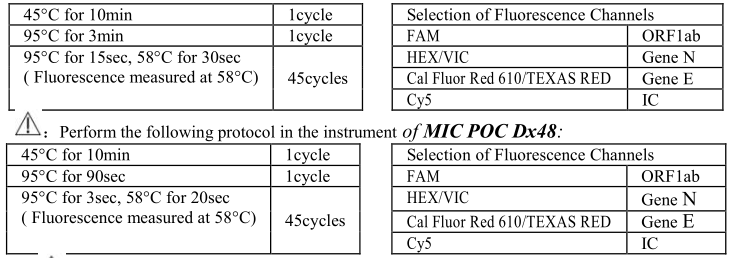
5) If you use ABI Prism ® system, please choose “none” as passive reference and quencher.
10. Threshold Setting: Just above the maximum level of Negative Control.
11. Quality Control: Negative Control and Positive Control must be performed correctly; otherwise
the sample results are invalid.
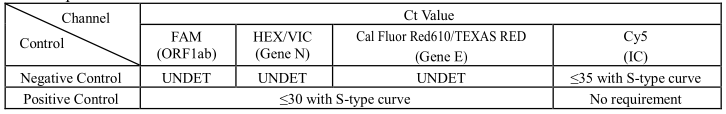
12. Data Analysis and Interpretation
The table below lists the expected results for the Novel Coronavirus (2019-nCoV) Real Time
Multiplex RT-PCR Kit (Detection for 3 Genes). If results obtained do not follow these guidelines,
contact Liferiver for consultation.
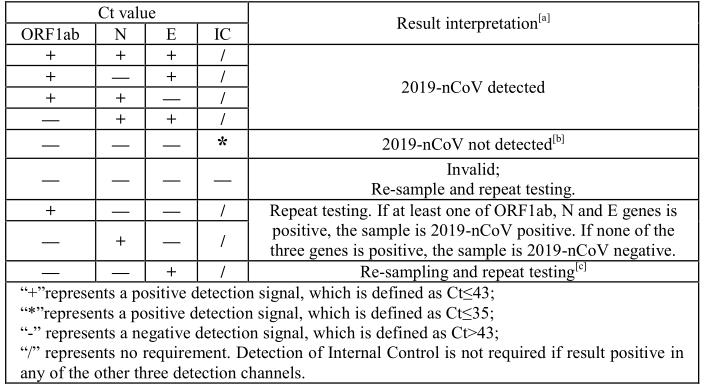
Note:
[a] Laboratories should report their diagnostic result as appropriate and in compliance with their
specific reporting system.
[b] Optimum specimen types and timing for peak viral levels during infections caused by 2019-nCoVhave not been determined. Collection of multiple specimens from the same patient may be necessary to detect the virus.
[c] If the results are positive for at least two of ORF1ab, N and E genes, the sample is 2019-nCoV
positive; If none of the three genes is positive, the sample is 2019-nCoV negative; If the result is
positive for ORF1ab or N gene, the sample is 2019-nCoV positive; If the result is still positive for only egene, the sample is 2019-nCoV positive or other near-source coronavirus positive (Once SARS virus and non-human samples are excluded, it can be reported as 2019-nCoV positive).
For further questions or problems,please contact our technical support at info@liferiverbiotech.com

 中文
中文 English
English Español
Español Français
Français